Sawai Launches Six Generic Drugs with 14 Strengths
Osaka, Japan – December 13, 2018 – Sawai Pharmaceutical Co., Ltd. (Sawai, Head office: Osaka, Japan, President: Mitsuo Sawai) today announced the launch of six compounds with 14 strengths of generic drugs. They will be launched sequentially beginning tomorrow. Sawai’s product line now includes 310* compounds with 756 strengths. Among the six compounds, four compounds, “Mirtazapine”, “Atomoxetine Hydrochloride”, “Tramadol Hydrochloride and Acetaminophen” and “Capecitabine”, will be launched for the first time as generics.
- TOARASET® Combination Tablets are Tramadol Hydrochloride and Acetaminophen, and are counted as one compound.
- FREWELL® Combination Tablets are Norethisterone and Ethinylestradiol, and are counted as one
The list of new products:
- MIRTAZAPINE OD Tablets 15 mg [SAWAI] and 30 mg [SAWAI], MIRTAZAPINE Tablets 15 mg [SAWAI] and 30 mg[SAWAI]
 ・Generic name: ・Generic name: |
Mirtazapine |
| ・Indications: | Depression and depressive state |
| ・Brand products: | REFLEX® TABLETS 15 mg and 30 mg,
REMERON® Tablets 15 mg and 30 mg |
| ・Striking features: | Orally disintegrating (OD) tablets are the first dosage form for
Mirtazapine. Sawai aimed to improve patient convenience by developing OD tablets which are taken at bedtime without water. In addition, “Product name” and “Strength” are printed on the tablets, allowing the product to be identified even after removing from blister packs. |
- ATOMOXETINE Capsules 5 mg [SAWAI], 10 mg [SAWAI], 25 mg [SAWAI] and 40 mg[SAWAI]
 ・Generic name:
・Generic name:Atomoxetine Hydrochloride ・Indications: Attention-deficit and hyperactivity disorder (ADHD) ・Brand products: Strattera® Capsules 5mg, 10 mg, 25 mg and 40 mg ・Striking features: The capsules are miniaturized and are printed with the “Generic
name” and “Strength”.
- TOARASET® Combination Tablets[SAWAI]
 ・Generic name: ・Generic name: |
Tramadol Hydrochloride and Acetaminophen |
| ・Indications: | Non-cancerous chronic pain and pain after tooth extraction that cannot be managed by treatments with non-opioid analgesics |
| ・Brand products: | TRAMCET® Combination Tablets |
 ・Striking features: ・Striking features: |
The “Product name” is printed on both sides of the tablet. “Therapeutic category”, “Product name” and “Generic name and Strength” are indicated on the blister pack. |
-
-
- ROPINIROLE Extended-release Tablets 2 mg [SAWAI] and 8 mg[SAWAI]
 ・Generic name:
・Generic name:Ropinirole Hydrochloride ・Indications: Parkinson’s disease ・Brand products: ReQuip® CR Tablets 2 mg and 8 mg
- ROPINIROLE Extended-release Tablets 2 mg [SAWAI] and 8 mg[SAWAI]
- FREWELL® Combination Tablets LD [SAWAI] and ULD [SAWAI]
-
 ・Generic name: ・Generic name: |
Norethisterone and Ethinylestradiol |
| ・Indications: | Dysmenorrhea |
| ・Brand products: | LUNABELL® Tablets LD and ULD |
| ・Striking features: | The blister pack can be set to the instruction card for easy disposal. |
- CAPECITABINE Tablets 300 mg [SAWAI]
 ・Generic name: ・Generic name: |
Capecitabine |
| ・Indications: | Inoperable or recurrent breast cancer, colorectal cancer, gastric cancer |
| ・Brand products: | XELODA® Tablets 300 |
| ・Striking features: | Sawai is launching the first generic of this product. Since anti- cancer treatment can be lengthy, generic drugs play an important role to help patients financially. |
A special feature of packages:
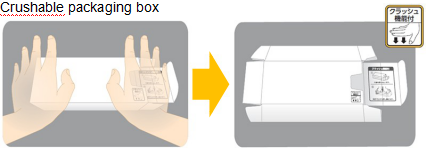
This box can be easily crushed upon disposal. This feature applies to MIRTAZAPINE OD Tablets, MIRTAZAPINE Tablets, and TOARASET® Combination Tablets
About Sawai
Founded in 1929, Sawai Pharmaceutical Co., Ltd. has grown into one of the leading generics companies in Japan. Guided by its corporate philosophy, “Always Putting Patients First,” Sawai markets more than 700 high-quality generic products and reliably delivers them to patients throughout Japan. In 2017, Sawai acquired US-based Upsher-Smith Laboratories, LLC marking its first step in overseas expansion to become a globally recognized generic pharmaceutical company. For more information, visit: https://www.sawai.co.jp/en/
The products announced in this press release are not approved by the Food & Drug Administration for sale and distribution in the United States.
For further information please contact:
PR/IR group, pr@sawai.co.jp

