Upsher-Smith Announces the Launch of Generic Clomipramine
UPSHER-SMITH ANNOUNCES THE LAUNCH OF GENERIC CLOMIPRAMINE
Maple Grove, MN – March 22, 2017 – Upsher-Smith Laboratories, Inc. (Upsher-Smith) today announced the U.S. launch of Clomipramine Hydrochloride Capsules* USP, 25 mg, 50 mg and 75 mg, the generic equivalent to Mallinckrodt Pharmaceuticals’ Anafranil® (clomipramine hydrochloride) Capsules USP. The clomipramine hydrochloride capsules market had U.S. sales of approximately $172 million for the 12 months ending December 2016, according to IMS Health. “Upsher-Smith has long been recognized within the pharmaceutical industry as a trusted name in generics,” said Rusty Field, President, Upsher-Smith. “This is an exciting time for the Company. Upsher-Smith currently has more active ANDA programs in development than at any point in its history and is committed to expanding its portfolio of quality, high-value generic products through both internal development and strategic partnerships.”
Product Information
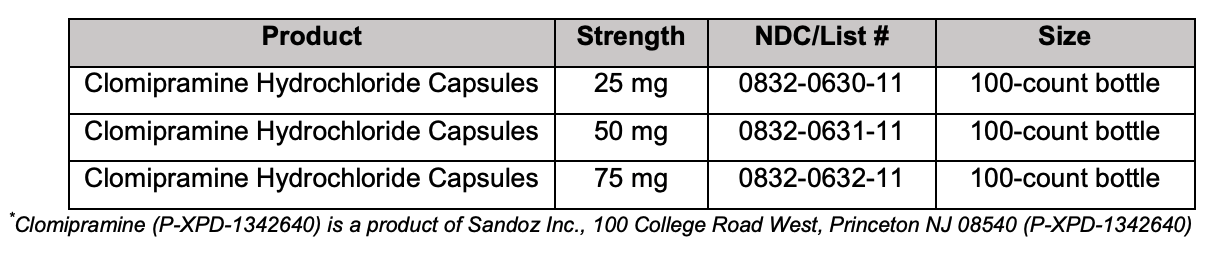
For questions about ordering Clomipramine Hydrochloride Capsules, please call UpsherSmith at 1-800-654-2299.
Suicidality and Antidepressant Drugs
Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of clomipramine hydrochloride or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Clomipramine hydrochloride is not approved for use in pediatric patients except for patients with obsessive compulsive disorder (OCD) (see accompanying full Product Package Insert for Warnings, Clinical Worsening and Suicide Risk, Precautions: Information for Patients and Precautions: Pediatric Use).
Please refer to the full Prescribing Information, including Boxed Warning for Clomipramine Hydrochloride Capsules. You can also visit call 1- 888-650-3789.
About Upsher-Smith
Upsher-Smith Laboratories, Inc., founded in 1919, is a growing, fully integrated pharmaceutical company dedicated to its mission of delivering high-value, high-quality therapies and solutions which measurably improve individuals’ lives. As a family-owned pharmaceutical company, we are able to adapt and thrive in a dynamic healthcare environment. Our world is constantly evolving, and we are continually adapting to the ever-changing needs of patients, physicians, pharmacists, and healthcare organizations. Where there is a need, we will work to deliver solutions that simplify access to treatment, deliver better health outcomes, and enhance life. Upsher-Smith has a particular focus on developing therapies for people living with central nervous system (CNS) conditions, such as seizure disorders.
Contact:
Elizabeth Likly
Kovak-Likly Communications
203-762-8833, elikly@klcpr.com

